Scope of registration
Diagnostic and screening procedures
Description
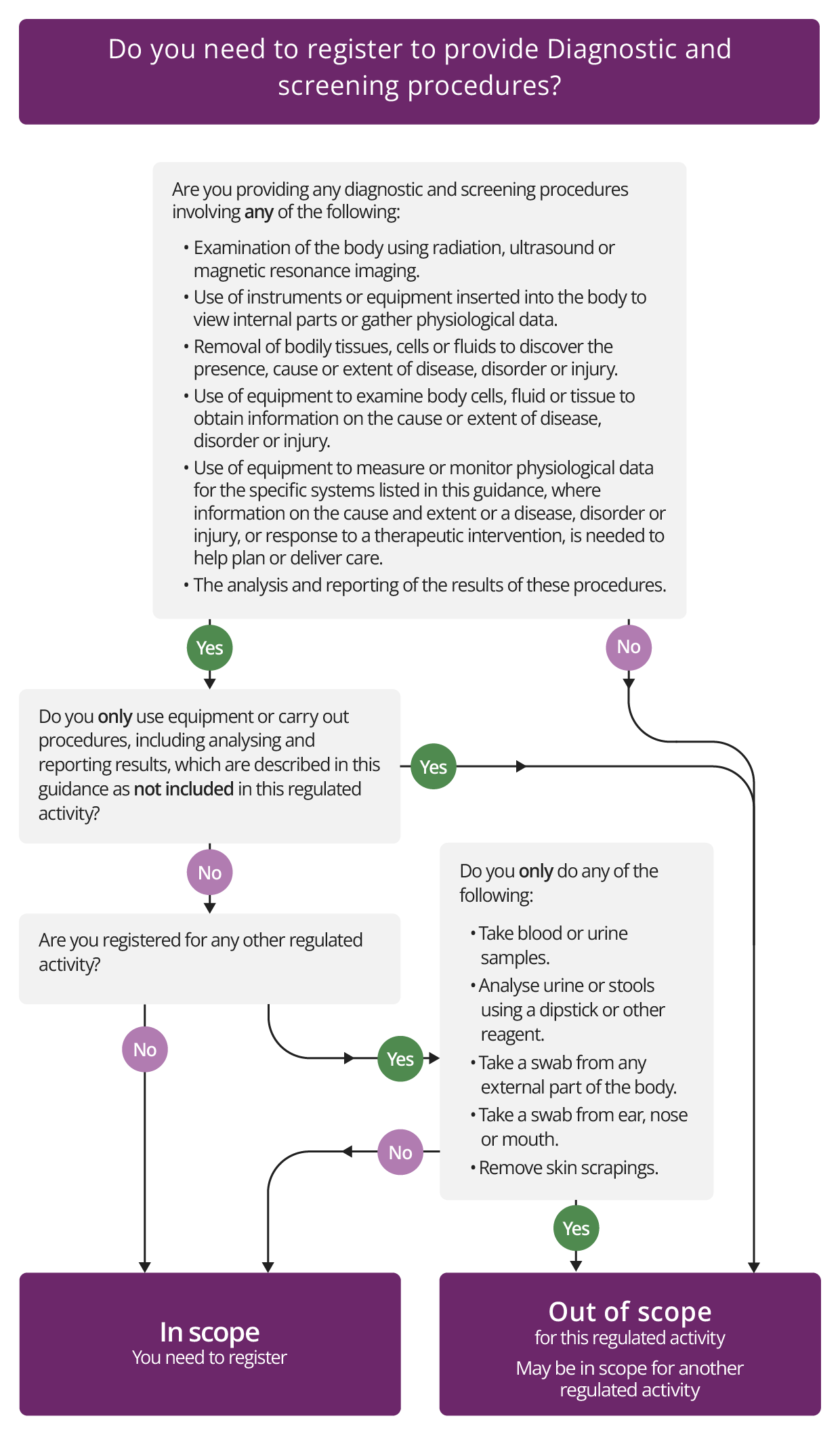
This regulated activity includes a wide range of procedures related to diagnostics, screening and physiological measurement.
It includes all diagnostic and screening procedures to examine the body that involve the use of any form of:
- radiation (including X-ray)
- ultrasound
- magnetic resonance imaging.
This includes all main forms of diagnostic radiology, radiography and sonography.
But it does not include use of the same technology when it is used for therapeutic purposes, such as radiotherapy or some forms of interventional radiology as these need to be registered for the activity of Treatment of disease, disorder or injury.
The activity of Diagnostic and screening procedures also includes the analysis and reporting of the examinations that are carried out.
If you use the X-ray, ultrasound or magnetic resonance imaging and you also carry out the analysis and reporting, both will be included within a single registration. But if you use a remote contractor for diagnostic analysis, the provider carrying out the analysis and reporting will also need to register in its own right.
Antenatal or baby scans
This activity is not just limited to scans carried out to get a diagnosis – it includes all procedures involving examination of the body by ultrasound, including antenatal ultrasounds scans. For example, an ultrasound performed on a pregnant person for the sole purpose of baby memorabilia or a keepsake (that is, not as part of the maternity pathway) is within the scope of this regulated activity, regardless of whether the scan is being carried out by a qualified sonographer or another person.
Subcontracting arrangements
Where diagnostic images are reported remotely by a subcontracted provider who is outside England, the subcontractor cannot register as they are outside of CQC's remit. However, we will hold to account the registered provider who made the contract with the subcontractor for the way the service is delivered and to make sure there are appropriate arrangements to deliver the service, including arrangements for quality assurance.
What the regulated activity includes
- Most forms of endoscopy. This is included because the activity covers procedures if they involve the use of instruments or equipment that are inserted into the body to:
- view inside of the body, or
- gather physiological data.
- Taking an intraoral scan. This is included because the activity involves the use of equipment inserted into the mouth to create a 3D visualisation of the teeth that can be used to:
- make dental appliances and construct restorations and prostheses
- help to diagnose orthodontic conditions and monitor the progress of treatment.
- Taking a sample or biopsy. This is included because the activity covers procedures if they involve removal of tissue, cells or fluids from the body, for the purpose of diagnosing disease, disorder or injury or monitoring its cause or extent. Therefore, anyone who 'removes' tissue, cells or fluids from the body for diagnostic reasons may need to register.
- Examining a sample. Anyone who uses equipment to examine tissue, cells or fluids from the body to obtain information on the cause and extent of a disease, disorder or injury may also need to register.
If you remove the sample and also carry out the examination, then you can include both in a single registration for the activity. But if you use a remote contractor for diagnostic analysis, such as a laboratory company, that provider will also need to register in its own right. - Physiological measurement (the use of equipment to measure or monitor physiological data). This means obtaining information on the causes and extent of a disease, disorder or injury, or the response to a therapeutic intervention, where the information is needed to plan and deliver care or treatment. It relates to the following systems:
- audio-vestibular
- vision
- neurological
- cardiovascular
- respiratory
- gastro-intestinal
- urinary.
Diagnostic services providing physiological measurement provide a wide range of specialist investigations and procedures that are often an essential part of care and treatment for patients. As well as assessing the function of major organ systems, physiological measurement includes measurement and tests that are part of normal clinical care when carrying on other regulated activities that a provider will already be registered for under the Health and Social Care Act 2008.
What the regulated activity does not include
You will not need to register for this regulated activity if you use:
- an auroscope
- a 12-lead electrocardiograph recording (ECG)
The following physiological tests are not included within the definition of physiological measurement, so you will not need to register if you carry out:
- pulse oximetry when used for 'spot' recording
- peak expiratory flow measured by a peak flow meter
- screening or non-diagnostic spirometry
- non-ambulatory blood pressure recording
- a hearing needs assessment or supply and fit a hearing aid if you are a hearing aid dispenser or are acting under the direction or supervision of a hearing aid dispenser, where:
- the person is aged 19 or over, or
- the person is under 19 and the procedure is carried out in, or arranged by, a school or 16 to 19 Academy.
The following are also excepted from this activity (see the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014, Schedule 1(7)(4)):
- Procedures carried out for research or analysing and reporting such procedures. However, this exception only applies where those research procedures do not form part of a person's care or treatment. As an example, a university with an imaging department that carries out research would need to register with us if it carries out a radiological examination for research purposes that is part of a patient’s care or treatment pathway.
- Taking X-rays by registered chiropractors or the use of ultrasound by registered physiotherapists.
- Carrying out procedures as part of some national cancer screening programmes.
- Fitness screening procedures in a gymnasium, related to the use of fitness equipment or fitness activities, (but treadmill tests for clinical purposes are not exempt).
- Blood tests carried out using a pin prick test or removing blood from a vein where the sample is not sent to a laboratory to be analysed.
- Taking urine samples where the sample is not sent to a laboratory to be analysed.
- Taking and analysing wound swabs.
- Sending samples of body fluids to a laboratory to be analysed, where a provider does not take the samples. For example, when a person produces a urine sample and gives it to a provider, and the provider then sends it away to be tested.
- Procedures carried out by a person in connection with any of the activities authorised by a licence granted by the Human Fertilisation and Embryology Authority.
- Taking or analysing samples of tissue, cells or fluids in order to determine the existence of a genetically inherited disease or disorder, or to determine the influence of a person’s genetic variation on their response to a drug. But these tests are not exempt if carried out as part of:
- planning or delivering the person’s treatment or care, or
- a national screening programme apart from a national cancer screening programme.
You can register for this regulated activity as well as any number of other activities. You do not have to be a healthcare professional to register for this activity.
We will consider certain low-risk procedures as part of a provider's overall registration with us. So, if you are registered with us for any other regulated activity, you will not have to register for the activity of Diagnostic and screening procedures just because you carry out the following procedures:
- taking blood or urine samples
- analysing urine or stools using a dip stick or other reagent
- taking a swab from any external part of the body or from the mouth, ear, nose or throat
- removing skin scrapings.
Organisations providing artificial intelligence software for clinical use
Some organisations that provide artificial intelligence (AI) software will need to register for this regulated activity. It is the use of this technology that may be a regulated activity, rather than the supply of the technology.
A healthcare provider that uses AI technology to deliver a regulated activity will be carrying on the regulated activity and will need to be registered with CQC. However, in some cases, the technology supplier may use its own technology to deliver a regulated activity, independently of any CQC-registered healthcare provider, and will need to register. This is becoming common in diagnostics and screening services, where a healthcare provider sends X-ray, CT or MRI images to an AI supplier that then uses its own AI to analyse the images and report the results. If the healthcare provider does not review the results of the analysis independently, then the AI supplier is likely to be carrying on the regulated activity and will need to register.
Suppliers of AI technology do not need to register with CQC where they only supply their technology to others.
Check if you need to register for Diagnostic and screening procedures