Scope of registration
Management of supply of blood and blood-derived products
Description
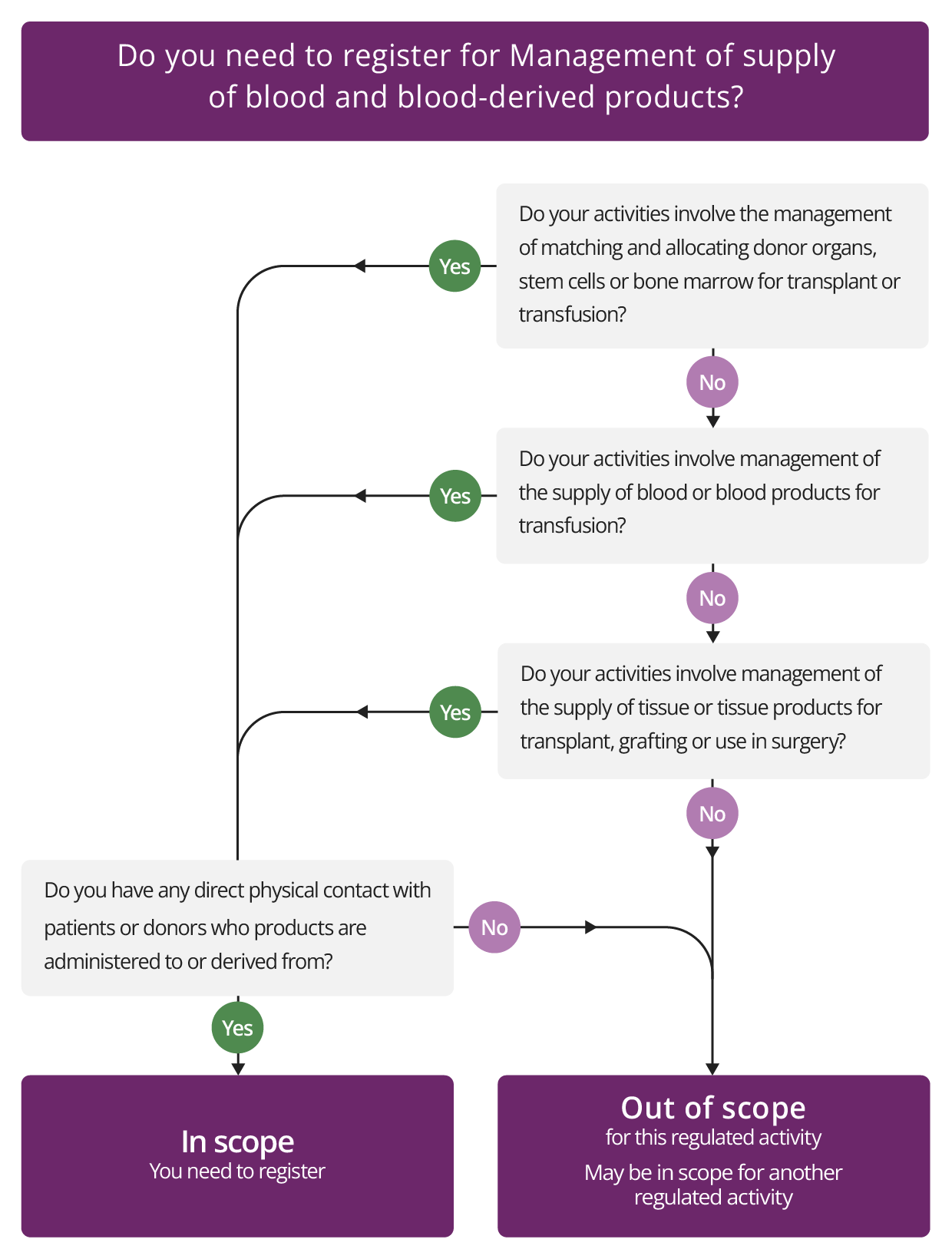
This regulated activity covers the management of:
- The supply of blood, blood components and blood-derived products for transfusion. Some examples include:
- where NHS Blood and Transplant manages the supply of blood
- a provider managing the supply of blood to another provider
- a service from a dedicated unit, such as a central or regional facility set up to provide this service to individual hospitals in a corporate group.
- The supply of tissues or tissue-derived products for transplant, grafting or use in surgery. For example, this will include supply of organs or tissue by NHS Blood and Transplant or any other provider of transplant organs.
- The matching and allocation of donor organs, stem cells or bone marrow for transplant or transfusion. For example, this will include the role of NHS Blood and Transplant or any other organisation that is involved in managing the supply of donor organs.
What this regulated activity does NOT include:
- The management of supply of blood, blood components and blood-derived products for transfusion, and tissue or tissue-derived products for transplant, grafting or use in surgery where it does not involve direct physical contact with patients or donors.
- How the products are stored, accessed and used in a hospital. This activity is about how blood and tissue products are supplied. Having appropriate equipment and supplies, and storing them, will be part of other regulated activities such as Treatment of disease, disorder or injury or Surgical procedures, rather than an activity in its own right.
- Providing taxi services or other forms of transport that transports blood, organs or tissue products between providers.
- Autologous transplant, where tissue is taken from a person and stored in order to be implanted back into them later. For example, a dental provider removing and storing tissue or bone from a patient and re-implanting it into the same patient at a later date. It also does not include autologous blood transfusion.
- Situations in which a provider’s role is only to remove an organ where the patient has chosen to be a donor. Removing an organ from a donor would be registerable under the regulated activity of Surgical procedures. In this case, a separate agency such as NHS Blood and Transplant will be responsible and would need to be registered for the onward supply of the organ to the transplantation service provider.
- In relation to donor organs, stem cells or bone marrow, the activity covers all of the supply procedures, from donation to matching and allocation, but does not cover the organ demand procedures, such as managing requests or waiting lists for transplantation.
Check if you need to register for Management of supply of blood and blood-derived products